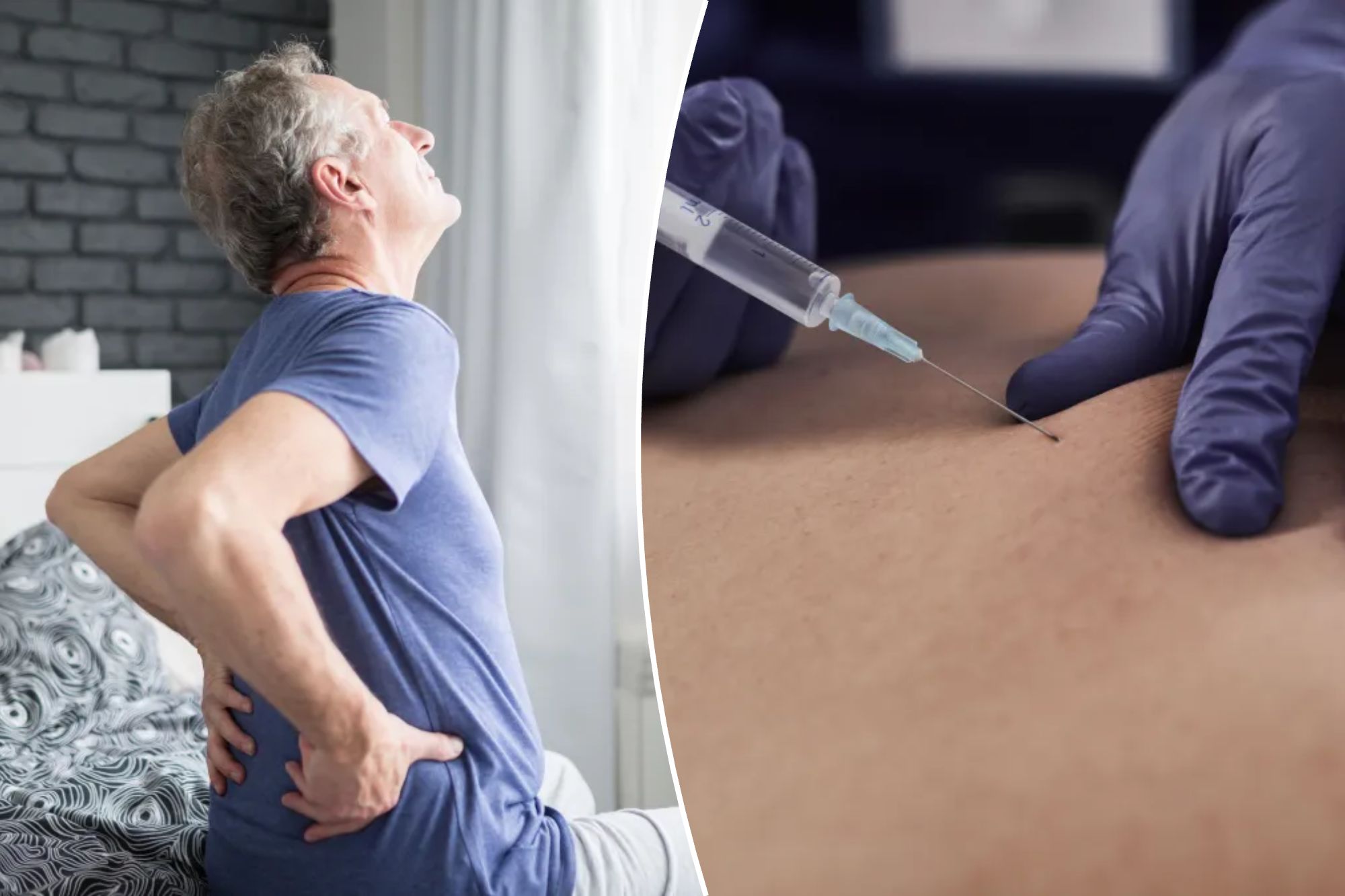
Chronic lower back pain is one of the main complaints that sends Americans to their doctors – and is a leading cause of lost days of work and disability claims.
While sliding discs, arthritis and spinal problems are often blamed, for some, the true culprit is an infection. Now, there is hope on the horizon for these patients.
Early clinical test results show that a new antibiotic drug can treat – or even cure – infection. Experts are greeting it as a “mass gamechanger” with the potential to drastically improve the quality of life for those suffering from chronic back pain.
Research suggests that for about 25% of patients with chronic back pain, the cause is a bacterial infection in their sponges – spongy tissue that pillows and absorb the shock among the vertebrae.
This infection damages the bones nearby, irritates the nerves and fuels of inflammation, leading to persistent back pain. It also causes changes in bone marrow, known as modic changes, which can be detected through an MRI scan.
UK -based biotechnical company Persica Pharmaceuticals has developed a drug that uses antibiotics to target and eliminate infection, aiming to address the root cause of pain than simply mask the symptoms.
Treatment, called PP353, combines three widely used substances: linezolid, an antibiotic; Iohexol, a contrast agent; and a thermosensitive gel that helps distribute the drug directly to the site of infection.
To prove its effectiveness, Persica recorded 44 patients from the UK, Spain, Denmark and New Zealand. All participants had suffered from severe back pain for at least six months – some for more than five years – and had modic changes. Their conditions were not improved with standard non-surgical treatments, such as physical therapy or painkillers.
Patiento patient received two injections of PP353 four days away on their lower back. The results were promising: six in 10 participants reported “significant” improvements, including reducing pain and disability.
Remarkably, patients continued to show improvement even 12 months after treatment. Moreover, PP353 was well tolerated, with no serious gastrointestinal side effects.
Dr. Joshua A. Hirsch, an intruder neuroradiologist at Massachusetts General Hospital and American Medical Advisor to Persika, praised the early promising drug results.
“In this early study, the treatment wing did significantly better than the placebo essentially all the parameters that were studied. Perhaps even more exciting, the impact seems to become better over time,” he said.
“If they arise in larger studies, implications for patients with back pain are potentially very significant,” Hirsch added.
Plus, as PP353 is not an opioid, it can provide an alternative to patients with chronic back pain seeking to avoid dependence sedatives between the constant opioid crisis in the JBA
However, it is likely to be some time – if at all – before the medicine is in the hands of patients.
The random clinical trial had a small sample size, so further testing is needed, and the drug must be approved by federal regulators before it could hit the market.
Persica is currently working at the start of a larger trial.
However, experts say early results are promising and suggest that treatment one day can have a profound impact on patients’ lives and society in general.
“If we can get these 25% of patients with low chronic back pain at work, again without medication, no more disability, then I think [that] It will be massive gamechanger for the future, ”said Dr. Shiva Tripathi, a painful of pain in the National Health Service and the chief of the trial investigator, The Guardian told.
#antibiotic #game #shift #chronic #pain
Image Source : nypost.com